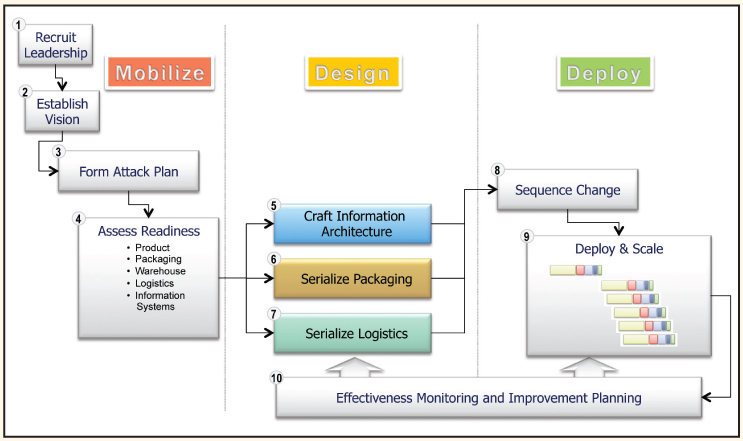
Enabling Item Level Serialization: Ten Key Steps to Ensure Success
Item level serialization (ILS) and traceability fosters supply chain integrity.
Whether to expose counterfeits, identify sources of product diversion, improve integrity in the reconciliation of returns or comply with increasing regulation, the ability to establish uniqueness at the item level provides unparalleled supply chain accountability and visibility benefits.
Attaining a common, yet integrated, approach to ILS requires participation and consensus of diverse stakeholders. Consensus building across divisions, geographic regions and corporate structures, is difficult to manage and control.
Adherence to a comprehensive, disciplined approach is necessary to clarify objectives, align priorities and facilitate a successful project outcome.
Consider these ten steps (Pharmaceutical Commerce website) when establishing your ILS and traceability program.



