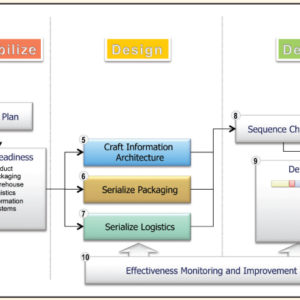
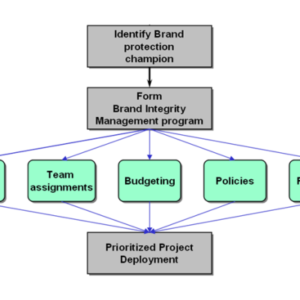
Enabling Item Level Serialization: Ten Key Steps to Ensure Success
Item level serialization (ILS) and traceability fosters supply chain integrity. Whether to expose counterfeits, identify sources of product diversion, improve integrity in the reconciliation of