Published FDA-UDI Ruling
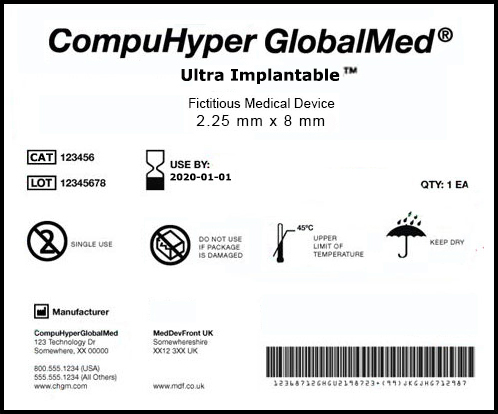
FDA Rule for Unique Device Identification System
The Food and Drug Administration (FDA) issued a final rule on October 24, 2013 to establish an identification system for medical devices.
Here is a brief summary of the rule:
- The label and packaging of medical devices must include a Unique Device Identifier (UDI).
- The labeler must submit product information concerning devices to FDA’s Global Unique Device Identification Database (GUDID).
- Each UDI must be provided in a plain-text version and in a form that uses Automatic Identification and Data Capture (AIDC) technology.
- The UDI will be required to be directly marked on the device itself if the device is intended to be used more than once and requires reprocessing before each use.



