Blog
UDI Regulation Released for Medical Devices
The FDA issued a long-awaited rule on Friday requiring companies to include Unique Device Identifiers, or UDIs, on medical devices. Each UDI will be entered into a database that the agency will maintain as a publicly searchable reference, allowing regulators to track the products, monitor them for safety and expedite recalls.
National Track&Trace Legislation Delayed Until After August Recess
Posted on August 2nd, by Gary Lerner in News, Pharmaceuticals.
The U.S. Senate adjourned this week without passing the Pharmaceutical Quality, Security and Accountability Act (S. 959).
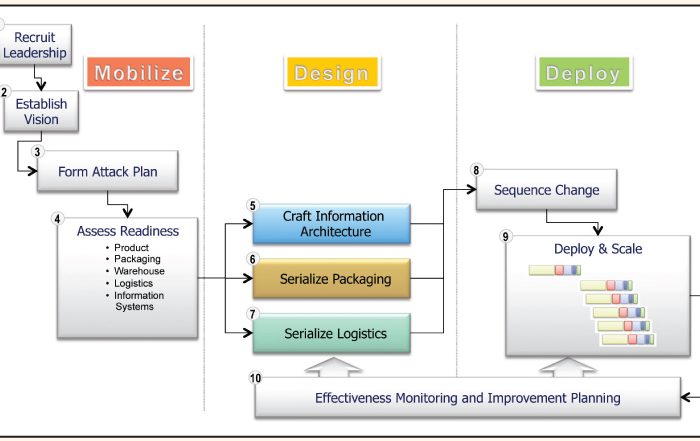
Enabling Item Level Serialization: Ten Key Steps to Ensure Success
Posted on August 1st, by Gary Lerner in Food & Beverage, Integrity Matters, Medical Devices, Pharmaceuticals.
Item level serialization (ILS) and traceability fosters supply chain integrity. Attaining a common, yet integrated, approach to ILS requires participation and consensus of diverse stakeholders. Consider these ten steps when establishing your ILS and traceability program.