Blog
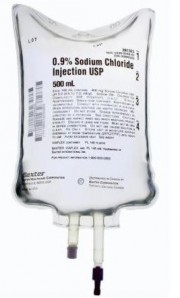
Shortage of Saline Solution may Threaten Hospital Patients
The FDA recently warned health care providers of a shortage of saline solution used for intravenous (IV) solutions, such as 0.9% sodium chloride injection. A number of factors are contributing to the shortage including a recall of multiple lots of Saline and high demand by hospitals, possibly triggered by patients exposed to the flu.
Drug Supply Chain Security Act Passes
On the eve of Thanksgiving 2013, a decade’s old struggle to better secure the US drug supply chain was quietly signed into law. The 113th Congress of the United States passed the Drug Quality and Security Act (DQSA) (House Bill 3204 which is now Public Law No: 113-54).
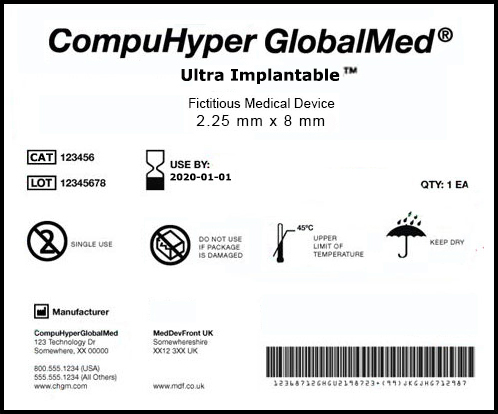
Published FDA-UDI Ruling
The Food and Drug Administration (FDA) issued a final rule on October 24, 2013 to establish an identification system for medical devices.
There are a number of important changes that will impact medical device manufacturers. See full text of the regulation here >>